Radiometric Age Dating Tutorial
·
Unstable naturally occurring isotopes emit particles in a
process known as radioactive decay
·
Radioactive decay occurs at known rates and using this you
can determine the age of certain types of rocks.
·
Read the course notes (section #3) for the complete theory
Principles
of radioactive dating
·
Parent: unstable radioactive isotope
·
Daughter product: results from the decay of a parent
·
Half-life: the time required for one-half (50%) of the
parent to change to daughter product
·
Comparing the ratio of parent to daughter yields the age of
the sample
1)
Examples of Common Half-lives
Parent Daughter Half-life
Rubidium 87 Strontium87 48.8 billion yrs
Thorium 232 Lead 208 14 billion years
Uranium 238 Lead
206 4.47
billion years
Potassium 40 Argon
40 1.25 billion yrs
Uranium 235 Lead
207 704 million years
Carbon 14 Nitrogen
14 5730 years
2) Chart
for half-lives elapsed
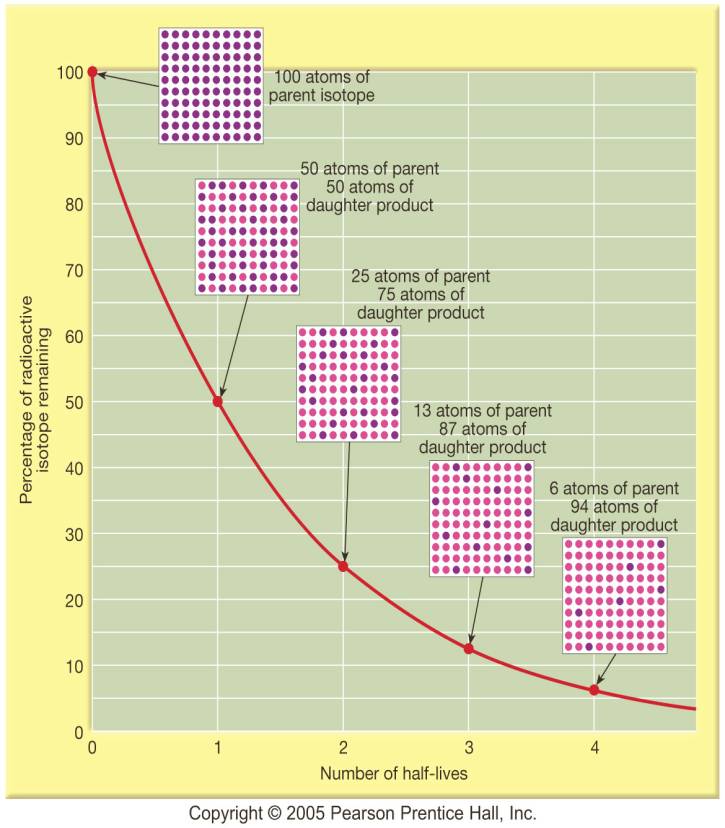
How it
really works
You need to have:
1. The
half-life of the material (above)
2. The number
(or fraction) of half-lives that have elapsed. This starts with a ratio of
parent to daughter, then using the chart you get the half-lives elapsed.
Then you multiply #1 times
#2.
Examples:
a. 50%
Uranium 238 and 50% Lead 206
4.47 Billion Years x 1
(half-life) = 4.47 Billion Yrs
b. 25%
Uranium 238 and 75% Lead 206
4.47 Billion Years x 2
(half-lives) = 8.94 Billion Yrs
c. 70% C14
and 30% N14
5730 years x 1/2 (of a
half-life) = 5730/2 or 2865 yrs
d. 84% C14
and 16% N14
5730 years x 1/4 or 0.25
(of a half-life) = 5730/4 or 1433 yrs
You have to estimate this
one for the half-lives-elapsed